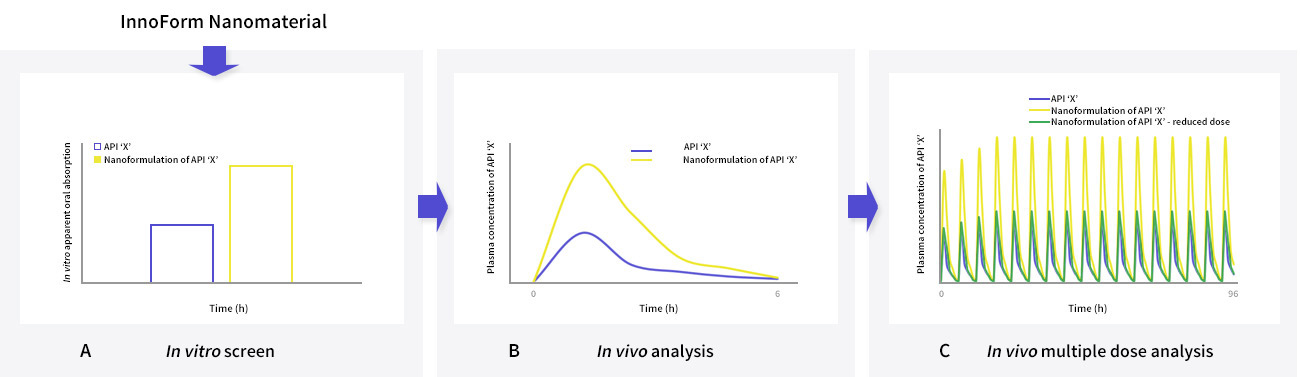
Potential pharmacological development of an InnoForm nanomaterial of API ‘X’ and an equivalent aqueous preparation using (A) an in vitro transwell model and (B) a pre clinical in vivo model, following a single oral dose. (C) Dose reduction strategy following multiple-doses in a pre clinical in vivo model. Examples of the methods employed and results for different APIs are described in McDonald et al., 2014; Giardiello et al 2016 and Savage et al., 2019.
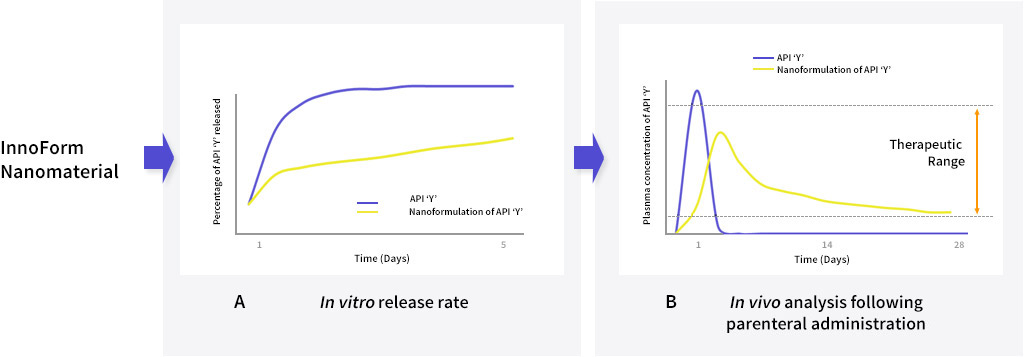
Potential pharmacological development of an InnoForm nanomaterial of API ‘Y’ for long-acting parenteral (subcutaneous or intramuscular) delivery. (A) in vitro release rate of API ‘Y’ performed in biologically relevant simulating buffers (B) plasma exposure of API ‘Y’ in a pre-clinical in vivo model, following a single parenteral injection. Examples of the methods employed and results for different APIs are described in Bakshi et al., 2018; Tatham et al., 2019 and Hobson et al., 2019.
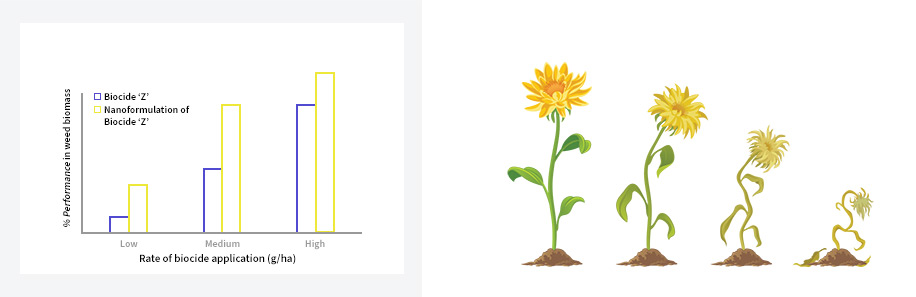
Potential glasshouse/field trial of an InnoForm nanomaterial of Agrochemical ‘Z’ following spray application. Previous studies have shown improved biocidal activity of InnoForm prepared agrochemicals and biocides and the potential for reduced dose or application rate.
Reference links
McDonald et al ., 2014
Giardiello et al., 2016
Savage et al., 2019
Bakshi et al., 2018
Tatham et al., 2019
Hobson et al., 2019
pubs.rsc.org/en/content/articlelanding/2019/na/c9na00529c#!divAbstract
